Tert-Butyl chloride
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
2-chloro-2-methylpropane
|
|||
| Other names
1,1-dimethylethyl chloride
1-chloro-1,1-dimethylethane chlorotrimethylmethane trimethylchloromethane t-butyl chloride |
|||
| Identifiers | |||
|
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.007.334 | ||
| EC Number | 208-066-4 | ||
|
PubChem CID
|
|||
| RTECS number | TX5040000 | ||
| UN number | 1127 | ||
|
|||
|
|||
| Properties | |||
| C4H9Cl | |||
| Molar mass | 92.57 g/mol | ||
| Appearance | Colorless liquid | ||
| Density | 0.851 g/mL | ||
| Melting point | −26 °C (−15 °F; 247 K) | ||
| Boiling point | 51 °C (124 °F; 324 K) | ||
| Sparingly sol in water, miscible with alcohol and ether | |||
| Vapor pressure | 34.9 kPa (20 °C) | ||
| Hazards | |||
|
EU classification (DSD) (outdated)
|
Flammable (F) | ||
| R-phrases (outdated) | R12, R36/37/38 | ||
| S-phrases (outdated) | S7, S9, S16, S29, S33 | ||
| NFPA 704 | |||
| Flash point | −9 °C (16 °F; 264 K) (open cup) −23 °C (closed cup) |
||
| 540 °C (1,004 °F; 813 K) | |||
| Related compounds | |||
|
Related alkyl halides
|
tert-Butyl bromide | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
tert-Butyl chloride is the organochloride with the formula (CH3)3CCl. It is a colorless, flammable liquid. It is sparingly soluble in water, with a tendency to undergo hydrolysis to the corresponding tert-butyl alcohol. It is produced industrially as a precursor to other organic compounds.
When tert-butyl chloride is dissolved in water, it undergoes a hydrolysis to tert-butyl alcohol. When dissolved in alcohols, the corresponding t-butyl ethers are produced.
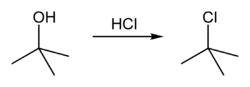
tert-Butyl chloride is produced by the reaction of tert-butyl alcohol with hydrogen chloride. In the laboratory, concentrated hydrochloric acid, is used. The conversion entails an SN1 reaction as shown below.
The overall reaction, therefore, is:
Because tert-butanol is a tertiary alcohol, the relative stability of the tert-butyl carbocation in the Step 2 allows the SN1 mechanism to be followed, whereas a primary alcohol would follow an SN2 mechanism.
tert-Butyl chloride is used to prepare the antioxidant tert-butylphenol and the fragrance neohexyl chloride.
...
Wikipedia