Ε-viniferin
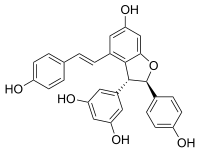 (−)-Trans-epsilon-viniferin
|
|
| Names | |
|---|---|
|
IUPAC name
5-[(2R,3R)-6-Hydroxy-2-(4-hydroxyphenyl)-4-[(E)-2-(4-hydroxyphenyl)ethenyl]-2,3-dihydro-1-benzofuran-3-yl]benzene-1,3-diol
|
|
| Other names
Viniferin
epsilon-Viniferin (−)-epsilon-Viniferin (−)-(E)-epsilon-viniferin trans-ε-viniferin (−)-Trans-epsilon-viniferin Iso-[epsilon]-viniferin cis-ε-viniferin Cis-epsilon-viniferin |
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C28H22O6 | |
| Molar mass | 454.47 g/mol |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
ε-Viniferin is a naturally occurring phenol, belonging to the stilbenoids family. It is a resveratrol dimer.
It is found in Vitis vinifera grapevines, in wines, in the Oriental medicinal plant Vitis coignetiae and in the stem bark of Dryobalanops aromatica.
Cis-epsilon-viniferin can be found in Paeonia lactiflora.
It shows a human enzymes inhibition activity.
Diptoindonesin A is a C-glucoside of ε-viniferin.
...
Wikipedia
