Biochanin A
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
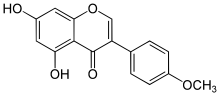
5,7-Dihydroxy-3-(4-methoxyphenyl)chromen-4-one
|
|
| Other names
Biochanin
4'-Methylgenistein olmelin Biochanine A Biochanin-A Genistein 4-methyl ether 5,7-Dihydroxy-4'-methoxyisoflavone |
|
| Identifiers | |
|
491-80-5 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:17574 |
| ChEMBL |
ChEMBL131921 |
| ChemSpider |
4444068 |
| ECHA InfoCard | 100.007.041 |
| 2829 | |
| KEGG |
C00814 |
| PubChem | 5280373 |
|
|
|
|
| Properties | |
| C16H12O5 | |
| Molar mass | 284.27 g·mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Biochanin A is an O-methylated isoflavone. It is a natural organic compound in the class of known as flavonoids. Biochanin A can be found in red clover in soy, in alfalfa sprouts, in peanuts, in chickpea (Cicer arietinum) and in other legumes.
Biochanin A is classified as a phytoestrogen and has putative benefits in dietary cancer prophylaxis. It has also been found to be a weak inhibitor of fatty acid amide hydrolase in vitro.
The enzyme biochanin-A reductase uses dihydrobiochanin A and NADP+ to produce biochanin A, NADPH, and H+. The enzyme isoflavone-7-O-beta-glucoside 6"-O-malonyltransferase uses malonyl-CoA and biochanin A 7-O-β-D-glucoside to produce CoA and biochanin A 7-O-(6-O-malonyl-β-D-glucoside).
...
Wikipedia
