Coelenterazine
 |
|
| Names | |
|---|---|
|
IUPAC name
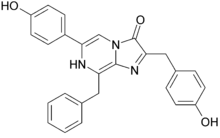
6-(4-hydroxyphenyl)-2-[(4-hydroxyphenyl)methyl]-8-(phenylmethyl)-7H-imidazo[1,2-a]pyrazin-3-one
|
|
| Other names
Coelenterazine, Renilla luciferin
|
|
| Identifiers | |
|
55779-48-1 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:2311 |
| ChemSpider |
2728 |
| ECHA InfoCard | 100.164.960 |
| PubChem | 2830 |
|
|
|
|
| Properties | |
| C26H21N3O3 | |
| Molar mass | 423.463 |
| Appearance | orange-yellow crystals |
| Melting point | 175 to 178 °C (347 to 352 °F; 448 to 451 K) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Coelenterazine is the luciferin, the light-emitting molecule, found in many aquatic organisms across seven phyla. It is the substrate of many luciferases such as Renilla reniformis luciferase (Rluc), Gaussia luciferase (Gluc), and photoproteins, including aequorin, and obelin.
Coelenterazine was simultaneously isolated and characterized by two groups studying the luminescent organisms sea pansy (Renilla reniformis) and the coelenterate Aequorea victoria, respectively. Both groups unknowingly discovered that the same compound was used in both luminescent systems, however the name of the molecule was given after the coelenterate. Likewise, the two main metabolites - coelenteramide and coelenteramine - were named after their respective functional groups. Despite being first discovered in Aequorea victoria, it was later shown that they do not synthesize coelenterazine, rather they obtain it through their diet, largely from crustaceans and copepods.
Coelenterazine is widely found in marine organisms including:
The compound has also been isolated from organisms that are not luminescent, such as the Atlantic herring and several shrimp species including Pandalus borealis and Pandalus platyuros.
Coelenterazine can be crystallized into orange-yellow crystals. The molecule absorbs light in the ultraviolet and visible spectrum, with peak absorption at 435 nm in methanol, giving the molecule a yellow color. The molecule spontaneously oxidizes in aerobic conditions or in some organic solvents such as dimethylformamide and DMSO and is preferentially stored in methanol or with an inert gas.
...
Wikipedia
