Disilene
 |
|
| Identifiers | |
|---|---|
| ChemSpider | |
|
PubChem CID
|
|
| Properties | |
| Si 2H 4 |
|
| Molar mass | 60.2028 g mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
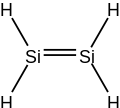
Disilene /daɪsaɪliːn/ (systematically named disilicon tetrahydride) is an inorganic compound with the chemical formula Si
2H
4. The name disilene, referring to the structure of a particular prototropic tautomer of the molecule. It is the simplest silene.
Disilene is a molecule with one Si=Si bond, and four equivalent Si-H bonds.
Unlike ethylene, disilene is kinetically unstable with respect to tautomerisation. Disilene has two other tautomers, that are very close in energy: (μ2-H)disilene, and disilanylidene.
Disilenes bearing sterically bulky substituents are isolable and have been well characterized although they remain mainly of academic interest. The first stabilised disilene was tetramesityldisilene, (C6Me3H2)4Si2. The Si=Si distance in this molecule is 2.15 Å, about 10% shorter than a typical Si–Si single bond. The Si2C4 core is roughly planar. Such species are typically prepared by reduction of organosilicon halides: 2 R2SiCl2 + 4 Na → R2Si=SiR2 + 4 NaCl An alternative synthesis involves photolysis of trisilacyclopropanes. When the R group is not bulky, cyclic or polymeric polysilanes are the products.
In one study a disilene is prepared by an intramolecular coupling of a 1,1-dibromosilane with potassium graphite. The silicon double bond in the resulting compound has a bond length of 227 picometer (second largest ever found) with trans-bent angles 33° and 31° (by X-ray diffraction).
...
Wikipedia
