Ezlopitant
 |
|
| Clinical data | |
|---|---|
| Routes of administration |
By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Excretion | Urine (32%), Feces (51%) |
| Identifiers | |
|
|
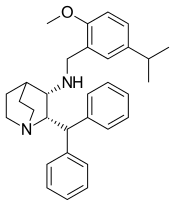
| Synonyms | CJ-11,974; (2S,3S)-2-Diphenylmethyl-3-[(5-isopropyl-2-methoxybenzyl)amino]quinuclidine |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C31H38N2O |
| Molar mass | 454.65 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Ezlopitant (INN, code name CJ-11,974) is an NK1 receptor antagonist. It has antiemetic and antinociceptive effects.Pfizer was developing ezlopitant for the treatment of irritable bowel syndrome but it appears to have been discontinued.
...
Wikipedia
