Ferritin
| Ferritin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
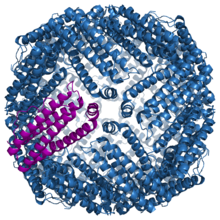
Structure of the murine ferritin complex 1lb3
|
|||||||||
| Identifiers | |||||||||
| Symbol | Ferritin | ||||||||
| Pfam | PF00210 | ||||||||
| Pfam clan | CL0044 | ||||||||
| InterPro | IPR008331 | ||||||||
| SCOP | 1fha | ||||||||
| SUPERFAMILY | 1fha | ||||||||
|
|||||||||
| Available protein structures: | |
|---|---|
| Pfam | structures |
| PDB | RCSB PDB; PDBe; PDBj |
| PDBsum | structure summary |
| ferritin, light polypeptide | |
|---|---|
| Identifiers | |
| Symbol | FTL |
| Entrez | 2512 |
| HUGO | 3999 |
| OMIM | 134790 |
| RefSeq | NM_000146 |
| UniProt | P02792 |
| Other data | |
| Locus | Chr. 19 q13.3–13.4 |
| ferritin, heavy polypeptide 1 | |
|---|---|
| Identifiers | |
| Symbol | FTH1 |
| Alt. symbols | FTHL6 |
| Entrez | 2495 |
| HUGO | 3976 |
| OMIM | 134770 |
| RefSeq | NM_002032 |
| UniProt | P02794 |
| Other data | |
| Locus | Chr. 11 q13 |
| ferritin mitochondrial | |
|---|---|
|
Crystallographic structure of mitochondrial ferritin.
|
|
| Identifiers | |
| Symbol | FTMT |
| Entrez | 94033 |
| HUGO | 17345 |
| OMIM | 608847 |
| RefSeq | NM_177478 |
| UniProt | Q8N4E7 |
| Other data | |
| Locus | Chr. 5 q23.1 |
Ferritin is a universal intracellular protein that stores iron and releases it in a controlled fashion. The protein is produced by almost all living organisms, including algae, bacteria, higher plants, and animals. In humans, it acts as a buffer against iron deficiency and iron overload. Ferritin is found in most tissues as a cytosolic protein, but small amounts are secreted into the serum where it functions as an iron carrier. Plasma ferritin is also an indirect marker of the total amount of iron stored in the body, hence serum ferritin is used as a diagnostic test for iron-deficiency anemia.
Ferritin is a globular protein complex consisting of 24 protein subunits forming a nanocage with multiple metal–protein interactions. It is the primary intracellular iron-storage protein in both prokaryotes and eukaryotes, keeping iron in a soluble and non-toxic form. Ferritin that is not combined with iron is called apoferritin.
Ferritin genes are highly conserved between species. All vertebrate ferritin genes have three introns and four exons. In human ferritin, introns are present between amino acid residues 14 and 15, 34 and 35, and 82 and 83; in addition, there are one to two hundred untranslated bases at either end of the combined exons. The tyrosine residue at amino acid position 27 is thought to be associated with biomineralization.
Ferritin is a hollow globular protein of 450 kDa consisting of 24 subunits that is present in every cell type. Typically it has internal and external diameters of about 8 and 12 nm, respectively. In vertebrates, these subunits are both the light (L) and the heavy (H) type with an apparent molecular weight of 19 kDa or 21 kDa respectively; their sequences are about 50% homologous. Amphibians have an additional ("M") type of ferritin; the single ferritin of plants and bacteria most closely resembles the vertebrate H-type. Two types have been recovered in the gastropod Lymnaea, the somatic ferritin being distinct from the yolk ferritin (see below). An additional subunit resembling Lymnaea soma ferritin is associated with shell formation in the pearl oyster. Two types are present in the parasite Schistosoma, one in males, the other in females. All the aforementioned ferritins are similar, in terms of their primary sequence, with the vertebrate H-type. In E. coli, a 20% similarity to human H-ferritin is observed. Inside the ferritin shell, iron ions form crystallites together with phosphate and hydroxide ions. The resulting particle is similar to the mineral ferrihydrite. Each ferritin complex can store about 4500 iron (Fe3+) ions.
...
Wikipedia
