Hexafluorosilicate
 |
|
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
Hexafluorosilicic acid
|
|
|
Systematic IUPAC name
Dihydrogen hexafluorosilicate
|
|
| Other names
Fluorosilicic acid, fluosilic acid, hydrofluorosilicic acid, silicofluoride, silicofluoric acid, oxonium hexafluorosilanediuide, oxonium hexafluoridosilicate(2−)
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.037.289 |
| EC Number | 241-034-8 |
|
PubChem CID
|
|
| RTECS number | VV8225000 |
| UN number | 1778 |
|
|
|
|
| Properties | |
| F6H2Si | |
| Molar mass | 144.09 g·mol−1 |
| Appearance | transparent, colorless, fuming liquid |
| Odor | sour, pungent |
| Density | 1.22 g/cm3 (25% soln.) 1.38 g/cm3 (35% soln.) 1.46 g/cm3 (61% soln.) |
| Melting point | ca. 19 °C (66 °F; 292 K) (60–70% solution) < −30 °C (−22 °F; 243 K) (35% solution) |
| Boiling point | 108.5 °C (227.3 °F; 381.6 K) (decomposes) |
| miscible | |
|
Refractive index (nD)
|
1.3465 |
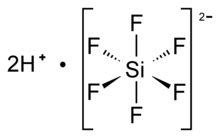
| Structure | |
| Octahedral SiF62− | |
| Hazards | |
| Safety data sheet | External MSDS |
|
EU classification (DSD) (outdated)
|
|
| R-phrases (outdated) | R34, R25 |
| S-phrases (outdated) | (S1/2), S26, S27, S45 |
| NFPA 704 | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
430 mg/kg (oral, rat) |
| Related compounds | |
|
Other cations
|
Ammonium hexafluorosilicate |
|
Related compounds
|
Hexafluorophosphoric acid Fluoroboric acid |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Hexafluorosilicic acid is an inorganic compound with the chemical formula (H
3O)
2SiF
6 (also written as (H
3O)
2[SiF
6]). It is a colorless liquid rarely encountered undiluted. Hexafluorosilicic acid has a distinctive sour taste and pungent smell. It is produced naturally on a large scale in volcanoes. It is manufactured as a precursor to aluminum trifluoride and synthetic cryolite. It is commonly used as a source of fluoride for water fluoridation. Salts derived from hexafluorosilicic acid are called hexafluorosilicates.
In aqueous solution, the hydronium cation (H3O+) is traditionally equated with a solvated proton, and as such, the formula for this compound is often written as H
2SiF
6. Extending that metaphor, the isolated compound is then written as H
2SiF
6·2H
2O.
...
Wikipedia

