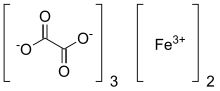
Iron(III) oxalate
 |
|
| Names | |
|---|---|
|
Systematic IUPAC name
iron(3+) ethanedioate (2:3)
|
|
| Other names
Iron(III) oxalate
|
|
| Identifiers | |
|
|
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.019.047 |
| EC Number | 220-951-7 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C6Fe2O12 | |
| Molar mass | 375.747 g/mol |
| Appearance | Pale yellow solid (anhydrous) Lime green solid (hexahydrate) |
| Odor | odorless |
| slightly soluble | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Ferric oxalate, also known as iron(III) oxalate, is a chemical compound composed of ferric ions and oxalate ligands; it may also be regarded as the ferric salt of oxalic acid. The anhydrous material is pale yellow; however, it may be hydrated to form Fe2(C2O4)3·6H2O which is bright green in colour
Like many oxalates, ferric oxalate has been investigated as a short term treatment for dentin hypersensitivity. It is used in certain toothpaste formulations; however, its effectiveness has been questioned.
It is used as the light-sensitive element in the Kallitype photographic printing process.
A number of other iron oxalates are known:-
...
Wikipedia
