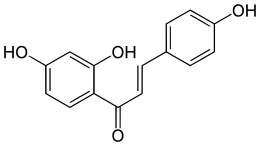
Isoliquiritigenin
 |
|
| Names | |
|---|---|
|
IUPAC name
(E)-1-(2,4-dihydroxyphenyl)-3-(4-hydroxyphenyl)prop-2-en-1-one
|
|
| Other names
6'-deoxychalcone
2',4,4'-Trihydroxychalcone 4,2',4'-Trihydroxychalcone 4'2'4'-trihydroxychalcone 2',4',4-Trihydroxychalcone |
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.202.617 |
| EC Number | 237-316-5 |
| KEGG | |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C15H12O4 | |
| Molar mass | 256.25 g/mol |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Isoliquiritigenin is a phenolic chemical compound found in licorice. It is under experimentation phase testing for use as a cancer treatment and as an aide for cocaine addiction. It is a sirtuin-activating compound.
The enzyme 6'-deoxychalcone synthase uses malonyl-CoA, 4-coumaroyl-CoA, NADPH, and H+ to produce CoA, isoliquiritigenin, CO2, NADP+, and H2O.
The enzyme isoliquiritigenin 2'-O-methyltransferase further transforms isoliquiritigenin into 2'-O-methylisoliquiritigenin.
Isoliquiritigenin has been found to potent (65 times higher affinity than diazepine) GABA-A benzodiapine receptor positive allosteric modulator.
...
Wikipedia
