Manganese(III) oxide
 |
|
| Names | |
|---|---|
| Other names
dimanganese trioxide, manganese sesquioxide, manganic oxide
|
|
| Identifiers | |
|
1317-34-6 |
|
| 3D model (Jmol) | Interactive image |
| ChemSpider |
14139 |
| ECHA InfoCard | 100.013.878 |
| PubChem | 14824 |
| RTECS number | OP915000 |
|
|
|
|
| Properties | |
| Mn2O3 | |
| Molar mass | 157.8743 g/mol |
| Appearance | brown or black crystalline |
| Density | 4.5 g/cm3 |
| Melting point | 888 °C (1,630 °F; 1,161 K) (alpha form) 940 °C, decomposes (beta form) |
| 0.00504 g/100 mL (alpha form) 0.01065 g/100 mL (beta form) |
|
| Solubility | insoluble in alcohol, acetone soluble in acid, ammonium chloride |
| +14,100·10−6 cm3/mol | |
| Structure | |
| Cubic, cI80 | |
| Ia-3, No. 206 | |
| Thermochemistry | |
|
Std molar
entropy (S |
110 J·mol−1·K−1 |
|
Std enthalpy of
formation (ΔfH |
−971 kJ·mol−1 |
| Hazards | |
| NFPA 704 | |
| Related compounds | |
|
Other anions
|
manganese trifluoride, manganese(III) acetate |
|
Other cations
|
chromium(III) oxide, iron(III) oxide |
|
Related compounds
|
manganese(II) oxide, manganese dioxide |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Manganese(III) oxide is a chemical compound with the formula Mn2O3.
Heating MnO2 in air at below 800 °C α-Mn2O3 is produced (higher temperatures produce Mn3O4). γ-Mn2O3 can be produced by oxidation followed by dehydration of manganese(II) hydroxide. Many preparations of nano-crystalline Mn2O3 have been reported, for example syntheses involving oxidation of MnII salts or reduction of MnO2.
Manganese (III) oxide is formed by the redox reaction in an alkaline cell:
Manganese (III) oxide Mn2O3 must not be confused with MnOOH manganese (III) oxyhydroxide. Contrary to Mn2O3, MnOOH is a compound that decomposes at about 300 °C to form MnO2.
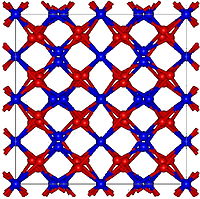
Mn2O3 is unlike many other transition metal oxides in that it does not adopt the corundum (Al2O3) structure. Two forms are generally recognized, α-Mn2O3 and γ-Mn2O3, although a high pressure form with the CaIrO3 structure has been reported too.
α-Mn2O3 has the cubic bixbyite structure, which is an example of a C-type rare earth sesquioxide (Pearson symbol cI80, space group Ia3, #206). The bixbyite structure has been found to be stabilised by the presence of small amounts of Fe3+, pure Mn2O3 has an orthorhombic structure (Pearson symbol oP24,space group Pbca, #61).
γ-Mn2O3 has a structure related to the spinel structure of Mn3O4 where the oxide ions are cubic close packed. This is similar to the relationship between γ-Fe2O3 and Fe3O4. γ-Mn2O3 is ferrimagnetic with a Néel temperature of 39 K.
...
Wikipedia

