Menthol
|
|
|||
 |
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
5-Methyl-2-(propan-2-yl)cyclohexan-1-ol
|
|||
| Other names
2-Isopropyl-5-methylcyclohexan-1-ol
2-Isopropyl-5-methylcyclohexanol 3-p-Menthanol Hexahydrothymol Menthomenthol Peppermint camphor |
|||
| Identifiers | |||
|
2216-51-5 (chiral) 89-78-1 (racemic) |
|||
| 3D model (Jmol) |
Interactive image Interactive image |
||
| ChEBI |
CHEBI:15409 |
||
| ChEMBL |
ChEMBL470670 |
||
| ChemSpider |
15803 |
||
| DrugBank |
DB00825 |
||
| ECHA InfoCard | 100.016.992 | ||
| 2430 | |||
| RTECS number | OT0350000, racemic | ||
| UNII |
BZ1R15MTK7 (chiral) YS08XHA860 (racemic) |
||
|
|||
|
|||
| Properties | |||
| C10H20O | |||
| Molar mass | 156.27 g·mol−1 | ||
| Appearance | White or colorless crystalline solid | ||
| Density | 0.890 g·cm−3, solid (racemic or (−)-isomer) |
||
| Melting point | 36 to 38 °C (97 to 100 °F; 309 to 311 K) racemic 42–45 °C, (−)-isomer, α crystalline form |
||
| Boiling point | 212 °C (414 °F; 485 K) | ||
| Slightly soluble, (−)-isomer | |||
| Hazards | |||
| Main hazards | Irritant, flammable | ||
| Safety data sheet |
See: data page External MSDS |
||
| R-phrases | R37/38, R41 | ||
| S-phrases | S26, S36 | ||
| Flash point | 93 °C (199 °F; 366 K) | ||
| Related compounds | |||
|
Related alcohols
|
Cyclohexanol, Pulegol, Dihydrocarveol, Piperitol |
||
|
Related compounds
|
Menthone, Menthene, Thymol, p-Cymene, Citronellal |
||
| Supplementary data page | |||
|
Refractive index (n), Dielectric constant (εr), etc. |
|||
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
||
| UV, IR, NMR, MS | |||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
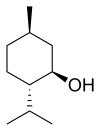
Menthol is an organic compound made synthetically or obtained from corn mint, peppermint, or other mint oils. It is a waxy, crystalline substance, clear or white in color, which is solid at room temperature and melts slightly above. The main form of menthol occurring in nature is (−)-menthol, which is assigned the (1R,2S,5R) configuration. Menthol has local anesthetic and counterirritant qualities, and it is widely used to relieve minor throat irritation. Menthol also acts as a weak kappa opioid receptor agonist.
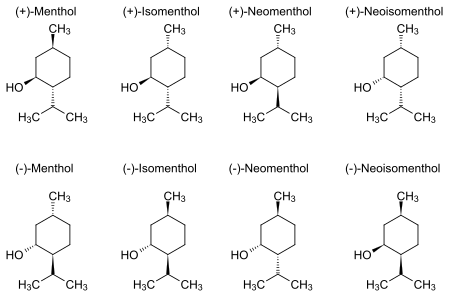
Natural menthol exists as one pure stereoisomer, nearly always the (1R,2S,5R) form (bottom left corner of the diagram below). The eight possible stereoisomers are:
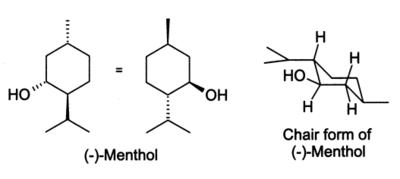
In the natural compound, the isopropyl group is in the trans orientation to both the methyl and hydroxyl groups. Thus, it can be drawn in any of the ways shown:
...
Wikipedia