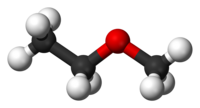
Methoxyethane
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Methoxyethane
|
|
| Other names
Methyl ethyl ether
Ethyl methyl ether |
|
| Identifiers | |
|
540-67-0 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:39832 |
| ChemSpider |
10441 |
| ECHA InfoCard | 100.128.000 |
| PubChem | 10903 |
|
|
|
|
| Properties | |
| C3H8O | |
| Molar mass | 60.10 g·mol−1 |
| Appearance | Colorless gas |
| Density | 0.7251 g cm−3 (at 0 °C) |
| Melting point | −113 °C (−171 °F; 160 K) |
| Boiling point | 7.4 °C (45.3 °F; 280.5 K) |
|
Refractive index (nD)
|
1.3420 (at 4 °C) |
| Viscosity | 0.224 cP at 25 °C |
| Hazards | |
| Main hazards | Extremely Flammable (F+), Liquefied gas |
| Safety data sheet | External MSDS |
| Related compounds | |
|
Related Ethers
|
Dimethyl ether Diethyl ether Methoxypropane |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Methoxyethane, also known as ethyl methyl ether, is an ethyl group with a bonded methoxy. Methoxyethane is a colorless gaseous ether with a medicine-like odor. It is extremely flammable, and its inhalation may cause asphyxiation or dizziness. As a Lewis base, it can react with Lewis acids to form salts and reacts violently with oxidizing agents.
...
Wikipedia
