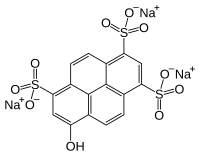
Pyranine
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
trisodium 8-hydroxypyrene-1,3,6-trisulfonate
|
|
| Other names
8-hydroxypyrene-1,3,6-trisulfonic acid, Solvent Green 7, HPTS, sulfonated hydroxy pyrene trisodium salt
|
|
| Identifiers | |
|
6358-69-6 |
|
| 3D model (Jmol) | Interactive image |
| ECHA InfoCard | 100.026.166 |
| EC Number | 228-783-6 |
| PubChem | 4136521 |
|
|
| Properties | |
| C16H7Na3O10S3 | |
| Molar mass | 524.37 |
| Appearance | yellow-green crystalline powder |
| Soluble | |
| Hazards | |
| Main hazards | XI |
| R-phrases | 36/37/38 |
| S-phrases | 26-36 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Pyranine is a hydrophilic, pH-sensitive fluorescent dye from the group of chemicals known as arylsulfonates. Pyranine is soluble in water and has applications as a coloring agent, biological stain, optical detecting reagent, and a pH indicator. One example would be the measurement of intracellular pH. Pyranine is also found in yellow highlighters, giving them their characteristic fluorescence and bright yellow-green colour. It is also found in some types of soap.
It is synthesized from pyrenetetrasulfonic acid and a solution of sodium hydroxide in water under reflux. The trisodium salt crystallizes as yellow needles when adding an aqueous solution of sodium chloride.
...
Wikipedia
