Tebbe reagent
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
μ-Chloro[di(cyclopenta-2,4-dien-1-yl)]dimethyl(μ-methylene)titaniumaluminum
|
|
| Other names
Tebbe reagent
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.157.162 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C13H18AlClTi | |
| Molar mass | 284.60 g/mol |
| Solubility in other solvents | toluene, benzene, dichloromethane, THF (low temperatures only) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
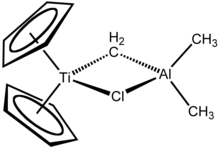
The Tebbe reagent is the organometallic compound with the formula (C5H5)2TiCH2ClAl(CH3)2. It is used in the methylenation of carbonyl compounds, that is it converts organic compounds containing the R2C=O group into the related R2C=CH2 derivative. It is a red solid that is pyrophoric in the air, and thus is typically handled with air-free techniques. It was originally synthesized by Fred Tebbe at DuPont Central Research.
Tebbe's reagent contains two tetrahedral metal centers linked by a pair of bridging ligands. The titanium has two cyclopentadienyl ([C
5H
5]−
, or Cp) rings and aluminium has two methyl groups. The titanium and aluminium atoms are linked together by both a methylene bridge (-CH2-) and a chloride atom in a nearly square-planar (Ti–CH2–Al–Cl) geometry. The Tebbe reagent was the first reported compound where a methylene bridge connects a transition metal (Ti) and a main group metal (Al).
The Tebbe reagent is synthesized from titanocene dichloride and trimethylaluminium in toluene solution.
...
Wikipedia
