Thallium(III) nitrate
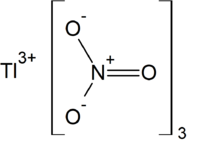 |
|
| Names | |
|---|---|
|
IUPAC name
thallium(3+) trinitrate
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| Tl(NO3)3 | |
| Molar mass | 390.398 |
| Appearance | colorless solid |
| Melting point | 103 °C (217 °F; 376 K) |
| Boiling point | decomposes |
| decomposes | |
| Hazards | |
| Safety data sheet | Mallinckrodt Baker |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Thallium(III) nitrate, also known as thallic nitrate, is a thallium compound with chemical formula Tl(NO3)3. It is normally found as the trihydrate. It is a colorless and highly toxic solid. It is a strong oxidizing agent useful in organic synthesis. Among its many transformations, it oxidizes methoxyl phenols to quinone acetals, alkenes to acetals, and cyclic alkenes to ring-contracted aldehydes.
...
Wikipedia
