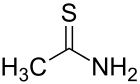
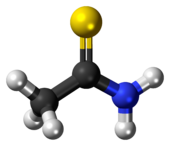
Thioacetamide
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Thioacetamide
|
|
|
Preferred IUPAC name
Ethanethioamide
|
|
| Other names
acetothioamide, TAA, thioacetimidic acid, TA, TAM
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.000.493 |
| KEGG | |
|
PubChem CID
|
|
| RTECS number | AC8925000 |
| UNII | |
|
|
|
|
| Properties | |
| C2H5NS | |
| Molar mass | 75.13 g/mol |
| Appearance | cristalls colourless crystals |
| Odor | slight mercaptan |
| Density | 1.269 g/cm3 |
| Melting point | 115 °C (239 °F; 388 K) |
| Boiling point | decomposes |
| good | |
| -42.45·10−6 cm3/mol | |
| Structure | |
| monoclinic | |
| Hazards | |
| Main hazards | Foul stench, carcinogenic |
| Safety data sheet | MSDS |
| R-phrases (outdated) | R22, R36, R37, R45 |
| S-phrases (outdated) | S45, S53 |
| Related compounds | |
|
Related compounds
|
acetamide, dithioacetic acid |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Thioacetamide is an organosulfur compound with the formula C2H5NS. This white crystalline solid is soluble in water and serves as a source of sulfide ions in the synthesis of organic and inorganic compounds. It is a prototypical thioamide.
Thioacetamide was widely used in classical qualitative inorganic analysis as an in situ source for sulfide ions. Thus, treatment of aqueous solutions of many metal cations to a solution of thioacetamide affords the corresponding metal sulfide:
Related precipitations occur for sources of soft trivalent cations (As3+, Sb3+, Bi3+) and monovalent cations (Ag+, Cu+).
Thioacetamide is prepared by treating acetamide with phosphorus pentasulfide as shown in the following idealized reaction:
The C2NH2S portion of the molecule is planar; the C-S and C-N distances are 1.713 and 1.324 Å, both indicating multiple bonding.
Thioacetamide is carcinogen class 2B. It is known to produce marked hepatotoxicity in exposed animals. Toxicity values are 301 mg/kg in rats (LD50, oral administration), 300 mg/kg in mice (LD50, intraperitoneal administration). This is evidenced by enzymatic changes, which include elevation in the levels of serum alanine transaminase, aspartate transaminase and aspartic acid.
...
Wikipedia
