Thymolphthalein
 |
|
| Names | |
|---|---|
|
IUPAC name
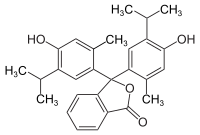
3,3-bis(4-hydroxy-2-methyl-5-propan-2-ylphenyl)-2-benzofuran-1-one
|
|
| Identifiers | |
|
125-20-2 |
|
| 3D model (Jmol) | Interactive image |
| ChEMBL |
ChEMBL587849 |
| ChemSpider |
29054 |
| ECHA InfoCard | 100.004.300 |
| EC Number | 204-729-7 |
| PubChem | 31316 |
|
|
|
|
| Properties | |
| C28H30O4 | |
| Molar mass | 430.54 g·mol−1 |
| Appearance | White powder |
| Melting point | 248 to 252 °C (478 to 486 °F; 521 to 525 K) (decomposes) |
| Hazards | |
| R-phrases | 4, 10 |
| S-phrases | S22 S24/25 |
| NFPA 704 | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Thymolphthalein is an acid–base (pH) indicator. Its transition range is around pH 9.3–10.5. Below this pH, it is colorless; above, it is blue. The molar extinction coefficient for the blue thymolphthalein dianion is 38,000 M−1 cm−1 at 595 nm.
Thymolphthalein is also known to have use as a laxative.
Thymolphthalein can be synthesized from thymol and phthalic anhydride by Friedel–Crafts alkylation:
...
Wikipedia

