Tolbutamide
 |
|
| Clinical data | |
|---|---|
| Trade names | Orinase |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682481 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
Oral (tablet) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 96% |
| Metabolism | Hepatic (CYP2C19-mediated) |
| Biological half-life | 4.5 to 6.5 hours |
| Excretion | Renal |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.000.541 |
| Chemical and physical data | |
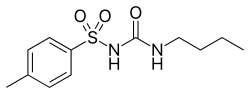
| Formula | C12H18N2O3S |
| Molar mass | 270.35 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Tolbutamide is a first-generation potassium channel blocker, sulfonylurea oral hypoglycemic medication. This drug may be used in the management of type 2 diabetes if diet alone is not effective. Tolbutamide stimulates the secretion of insulin by the pancreas.
It is not routinely used due to a higher incidence of adverse effects compared to newer, second-generation sulfonylureas, such as glyburide. It generally has a short duration of action due to its rapid metabolism, so is safe for use in older people.
It was discovered in 1956.
Salicylates displace tolbutamide from its binding site on plasma binding proteins which lead to increase in free tolbutamide concentration, thus hypoglycemic shock.
Orinase was developed by Upjohn Co. at a time when the primary medical treatment for diabetes was insulin injections. Eli Lilly had a lock on the market for insulin production at the time. Orinase, like other treatments for drugs detected by so-called paraclinical signs rather than clinically observable signs or patient-reported symptoms, benefitted from an increased sensitivity and availability of blood glucose testing. Milton Moskowitz (editor in 1961 of Drug and Cosmetic Industry) claimed the introduction of Orinase, "expanded the total market by bringing under medical care diabetics who were formerly not treated." It did this by changing the mindset about diabetes even more than insulin had. Treatment of this chronic disease was no longer seen as a mere slowing of "inexorable degeneration", but instead viewed through "a model of surveillance and early detection."
Orinase and other sulfonylureas emerged from European pharmaceutical research into antibiotics, specifically from attempts to develop sulfa compounds. One of the contenders for a new sulfa antibiotic had serious side effects during clinical trials at the University of Montpellier including blackouts, convulsions, and coma, side effects not observed with any other drugs in the sulfa cohort. An insulin researcher at the same university heard of these side effects and recognized them as common results of hypoglycemia. The resulting class of drugs for lowering blood sugar came to be known as the sulfonylureas, starting with Orinase and still in use today in other forms.
...
Wikipedia
