Vinyl flooring
 |
|
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
poly(1-chloroethene)
|
|
| Other names
Polychloroethylene
|
|
| Identifiers | |
| Abbreviations | PVC |
| ChEBI | |
| ChemSpider |
|
| ECHA InfoCard | 100.120.191 |
| KEGG | |
| MeSH | Polyvinyl+Chloride |
| Properties | |
| (C2H3Cl)n | |
| −10.71×10−6 (SI, 22°C) | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
| Elongation at break | 20–40% |
|---|---|
| Notch test | 2–5 kJ/m2 |
| Glass Transition Temperature | 82 °C (180 °F) |
| Melting point | 100 °C (212 °F) to 260 °C (500 °F) |
| Effective heat of combustion | 17.95 MJ/kg |
| Specific heat (c) | 0.9 kJ/(kg·K) |
| Water absorption (ASTM) | 0.04–0.4 |
| Dielectric Breakdown Voltage | 40 MV/m |
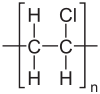
Polyvinyl chloride (/ˌpɒlivaɪnəl ˈklɔəraɪd/), also known as poly vinyl or vinyl, commonly abbreviated PVC, is the world's third-most widely produced synthetic plastic polymer, after polyethylene and polypropylene.
PVC comes in two basic forms: rigid (sometimes abbreviated as RPVC) and flexible. The rigid form of PVC is used in construction for pipe and in profile applications such as doors and windows. It is also used for bottles, other non-food packaging, and cards (such as bank or membership cards). It can be made softer and more flexible by the addition of plasticizers, the most widely used being phthalates. In this form, it is also used in plumbing, electrical cable insulation, imitation leather, signage, phonograph records, inflatable products, and many applications where it replaces rubber.
Pure polyvinyl chloride is a white, brittle solid. It is insoluble in alcohol but slightly soluble in tetrahydrofuran.
PVC was accidentally synthesized in 1872 by German chemist Eugen Baumann. The polymer appeared as a white solid inside a flask of vinyl chloride that had been left exposed to sunlight. In the early 20th century the Russian chemist Ivan Ostromislensky and Fritz Klatte of the German chemical company Griesheim-Elektron both attempted to use PVC in commercial products, but difficulties in processing the rigid, sometimes brittle polymer thwarted their efforts. Waldo Semon and the B.F. Goodrich Company developed a method in 1926 to plasticize PVC by blending it with various additives. The result was a more flexible and more easily processed material that soon achieved widespread commercial use.
...
Wikipedia
